4 min read
L2L and Dart Container Recognized as Finalists for the 2024 Manufacturing Leadership Awards
The partnership provided double-digit improvements to Dart’s OEE and set the stage for a pilot facility to enjoy its...
September 03, 2020
"Across the nation, medical device and medical supply manufacturers face a massive need for products to combat the COVID-19 pandemic. And, there is high demand for quick delivery. This puts manufacturers in a bind. Production speed is easy to increase if you are willing to sacrifice quality. Quality is easy to maintain if there’s enough time. To achieve the highest quality at higher production rates, there are strategies to apply.
Medical production is a no-fail environment, as defects or inaccuracies can cost lives. Communicating and escalating quality control (QC) problems must be a top priority, streamlined so they are clear to everyone involved.
Quality must be built into medical devices. Post-production inspections are vital, but they can’t be the only solution. In a crisis, there’s no time for do-overs.
If quality inspectors are finding defects, you have a quality control risk championed by a process capability problem. A zero defects philosophy means fully addressing every problem. Errors found during QC inspection should signal high alert. It should trigger a full root cause analysis and a rigorous process for addressing the issue and implementing corrective actions from the production process back to the supplier and the process if needed."

Apr 11, 2024by L2L
The partnership provided double-digit improvements to Dart’s OEE and set the stage for a pilot facility to enjoy its...

Feb 8, 2024by L2L
Kyle Petty to Keynote the Annual User Conference in May
SALT LAKE CITY, Utah, February 8, 2024 - L2L, the global...
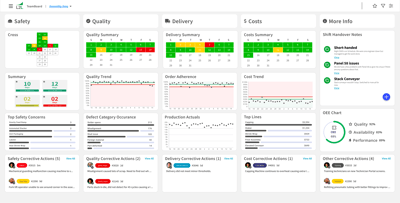
Sep 6, 2023by Trent Johnson
Salt Lake City, UT – September 6, 2023: L2L, the Connected Workforce pioneer, is excited to announce the highly...
What makes L2L so unique is the fact that the product was developed by real manufacturing users. People that truly understand the day-to-day issues and concerns that drive the production floor.
